不裝冷氣機的辦公室

台東深層海水低溫模廠大樓,沒裝設冷氣機,卻利用深層海水當冷媒,讓天然的冷氣格外舒適。

台東深層海水低溫模廠大樓,沒裝設冷氣機,卻利用深層海水當冷媒,讓天然的冷氣格外舒適。
連續幾天高溫,讓人受不了,辦公室的冷氣機不停運轉,還是無法消暑。不過,台東知本海邊水試所的深層海水低溫模廠大樓,完全找不到冷氣機,辦公室內卻一點也不熱。
「這種溫度比冷氣機還舒服」,辦公室警衛說,雖然沒有冷氣,但是裡面的溫度比清晨的微風還舒服,就像置身在森林的樹蔭下。
模廠大樓利用冷熱交換原理,將深層海水當作冷媒,降低淡水溫度,再接引到冷排吹風,變成天然的冷媒冷氣。
負責管理的水試所東部海洋生物研究中心副研究員何源興表示,大樓的冷氣空調不需要冷媒,也沒有風扇、壓縮機,僅利用冷熱交換原理,將淡水溫度降低,然後再以送風方式送到各辦公室。
何源興說,抽起來的深層海水溫度在攝氏10度左右,轉換淡水低溫時,可以達到攝氏13度的低溫,天然溫度,讓人心曠神怡。
除了天然冷氣外,也利用深層海水的低溫特性,透過布管,讓低溫的海水流經植物根部土壤,降低土壤溫度,栽種高山蔬菜、菇類、花卉、水果,也可促使植物停止休眠期、加速生長。
深層海水冰水機之介紹
http://dns.tpetube.com.tw/newsfiles/615/A17-29.pdf
台灣深層海水產業發展契機
資料來源 : 經濟部水利署
一、深層海水定義
「深層海水」於台灣本島之廣義定義如下:
指在斜溫層以下(約海平面200尺以下)的海水,陽光無法進入,具低溫(我國海域調查為6~9℃)、高營養鹽、清澈乾淨、少病原菌等特性。
台灣東部地區深層海水之優勢
台灣東部位於東亞大陸棚邊緣,距海岸3~5公里,水深即可達到1000公尺以上,具有取水路徑短,佈管與取水費用低等優勢。
台灣東部海面有黑潮帶來表層高溫暖水,與深層低溫冷水,溫差大,具有溫差發電與潔淨能源產業之發展優勢。
台灣地理位置優越,位居亞太地區樞紐,北連日韓,南接東協及印度,東邊為全球最大經濟體—美國,西邊緊鄰中國大陸,具有進軍東亞新興市場,行銷拓展至全球之優勢。
政府為促進東部產業發展,於政策面積極推動相關工作,包括資源調查、應用技術研發、產品檢驗認證、產業育成、行銷推廣等,以提供深層海水產業優良之投資環境。
三、深層海水國際發展情形
美國(夏威夷)深層海水產業發展
日本深層海水產業發展
韓國深層海水產業發展
http://www.cepd.gov.tw/att/0014185/0014185_21.pdf
深層海水其它妙用
水深超過200 米 的深海中的海水被稱之為深層海水,深層海水占所有海水量的93%,含量相當大。深層海水最大的特點——潔淨,深層海水未被現代產業廢水以及生活排水所污染。幾乎照不到陽光的深層海水不會發生光合反應,浮游生物處於休眠狀態。在世界各大海域,表層水正日益受到各種化學化合物以及環境激素的污染,但海洋的深層海水則是沒有污染的處女地。
科學研究表明:海洋中的污染物都被表層海水溶解了,所以深層海水未被污染;而且,深層海水中所含的細菌類非常少,僅為表層水的千分之一至萬分之一。因此,它是非常清潔的水源。
深層海水中含有豐富的氮、矽、磷等成分,可用於發展海水養殖業,用它養殖的海產品也不存在污染。如日本富山縣水產試驗場抽取深層海水用於人工飼養某些魚蝦類,大大提高了魚蝦成活率。除了用深層海水大量生產龍蝦和蝦苗,還可養殖名貴的鱒魚、鮃魚和海豚等,已取得了良好的成效。包括鐘紡、田邊制藥等日本大企業在內,已經有50 家企業利用這種海水開發出120 多種商品。他們還利用深層海水大量培養微細藻類作為健康食品的原料。
最近,海洋學家發現,深層海水受海底地形及氣象條件的影響,會自動湧升到海面上來。這種“湧升海面”雖然僅占全球海洋面積的幹分之一,卻集中了魚類資源的60%-70%。其奧秘是富含微量元素的深層海水湧上海面後,浮游生物和藻類又能得以生長,為魚類提供了餌食。
目前,深層海水在醫學領域也有了用武之地,現在它已成為一種奇妙的“綠色藥品”。如日本高知縣醫科大學醫生們用深層海水治療先天過敏性皮炎,有60% 的患者收到良好的效果。更為奇妙的是,潔淨的深層海水也引起了食品和化妝品生產廠家的極大興趣,準備研發出各類產品。
近年來,日本開發研製的一系列深層海水食品已種類繁多,如深層海水豆腐、醬油、果汁、酒類、瓶裝水、豆漿、果凍、糕點以及天然鹽等產品都一一登場。由於深層海水清潔少菌,且所含微量元素和礦物質幾乎是均衡狀態,因而深層海水生產的食品日益受到人們的青睞。其中日本的富山、高知等幾個縣已在海水養殖、康復美容、飲料食品等方面取得了一系列成果,使全球沿海居民受到啟迪。
海洋裏的深層海水每時每刻都在進行著蔚為壯觀的大循環,這種大循環使海洋充滿了活力,它也給21世紀的人類帶來了新的機遇,新的挑戰,新的研究,新的探索。
其他能源利用:
Southern California Edison Proposes Clean Hydrogen Power Generation to Reduce GHG Emissions
According to a press release dated May 17, Edison International’s (NYSE:EIX) electricity utility, Southern California Edison (SCE), has requested approval to build and test a commercial 600 MW power plant to determine the feasibility of a new combination of several advanced “clean” coal technologies in an effort to advance these emerging approaches to low-carbon generation.
Their proposal consist of:
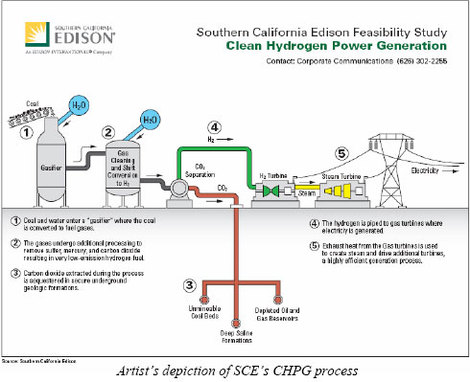
Their proposal consist of:
- A gasifier that combines coal and steam with a controlled amount of oxygen under high pressures to produce hydrogen and carbon monoxide.
- Converting the carbon monoxide to additional hydrogen and carbon dioxide in the shift conversion.
- Further processing the gasses to remove sulfur, mercury, and carbon dioxide.
- Sequestering the carbon in a depleted oil formation, enabling enhanced oil recovery, or in a deep saline formation.
- Producing a mostly hydrogen fuel, emitting only 10 percent of the carbon released by an integrated gasification combined-cycle coal project without carbon capture.
- The hydrogen is fed to gas turbines where electricity is generated.
- Exhaust heat from the gas turbines is used to create steam and drive additional turbines.
- The use of these technologies in a full-scale, 600-megawatt (MW) commercial generating facility.

The advanced technologies in SCE’s proposed study, an approach the utility calls Clean Hydrogen Power Generation (CHPG), are being considered or tested in clean coal projects elsewhere. The SCE plan would be the first assessment of a full-scale, 600-MW facility using all of them. One of the main differences between this process and others is that the carbon monoxide is converted to additional hydrogen enabling the gas turbines to only burn hydrogen.
SCE is seeking authorization to commit $52 million of revenues it collects from customer rates during a two-year period to an advanced technology feasibility study. If approved, this would represent less than a quarter of one percent of current customer rates.
SCE also is the nation’s leading purchaser of renewable energy, buying and delivering approximately 13 billion kWh in 2006 from wind, solar, biomass, biogas, geothermal, and small hydro suppliers – 16.7% of the power it delivered to customers. An equivalent amount of generation using fossil fuels would produce 7 million tons of GHG emissions. SCE purchases one-sixth of all U.S. renewable energy used to generate electricity for retail sale, including more than 90% of all the U.S. solar generation.
The above article was adapted from the press release referred to in the first paragraph.
氫氧能源
Hydrogen Energy (氫氧能源)

NASA uses hydrogen fuel to launch the space shuttles. Credit: NASA
Hydrogen is the simplest element. An atom of hydrogen consists of only one proton and one electron. It's also the most plentiful element in the universe. Despite its simplicity and abundance, hydrogen doesn't occur naturally as a gas on the Earth - it's always combined with other elements. Water, for example, is a combination of hydrogen and oxygen (H2O).
Hydrogen is also found in many organic compounds, notably the hydrocarbons that make up many of our fuels, such as gasoline, natural gas, methanol, and propane. Hydrogen can be separated from hydrocarbons through the application of heat - a process known as reforming. Currently, most hydrogen is made this way from natural gas. An electrical current can also be used to separate water into its components of oxygen and hydrogen. This process is known as electrolysis. Some algae and bacteria, using sunlight as their energy source, even give off hydrogen under certain conditions.
Hydrogen is high in energy, yet an engine that burns pure hydrogen produces almost no pollution. NASA has used liquid hydrogen since the 1970s to propel the space shuttle and other rockets into orbit. Hydrogen fuel cells power the shuttle's electrical systems, producing a clean byproduct - pure water, which the crew drinks.
A fuel cell combines hydrogen and oxygen to produce electricity, heat, and water. Fuel cells are often compared to batteries. Both convert the energy produced by a chemical reaction into usable electric power. However, the fuel cell will produce electricity as long as fuel (hydrogen) is supplied, never losing its charge.
Fuel cells are a promising technology for use as a source of heat and electricity for buildings, and as an electrical power source for electric motors propelling vehicles. Fuel cells operate best on pure hydrogen. But fuels like natural gas, methanol, or even gasoline can be reformed to produce the hydrogen required for fuel cells. Some fuel cells even can be fueled directly with methanol, without using a reformer.
In the future, hydrogen could also join electricity as an important energy carrier. An energy carrier moves and delivers energy in a usable form to consumers. Renewable energy sources, like the sun and wind, can't produce energy all the time. But they could, for example, produce electric energy and hydrogen, which can be stored until it's needed. Hydrogen can also be transported (like electricity) to locations where it is needed.
Additional Resources on Hydrogen Energy
- Find out about NREL's research in hydrogen and fuel cell technologies.
- Also see the U.S. Department of Energy's Hydrogen, Fuel Cells and Infrastructure Technologies Program
Water-Rock Reaction May Provide Enough Hydrogen 'Food' to Sustain Life in Ocean's Crust or On Mars
Mars surface. A chemical reaction between iron-containing minerals and water may produce enough hydrogen "food" to sustain microbial communities living in pores and cracks within the enormous volume of rock below the ocean floor and parts of the continents, according to a new study. The findings also hint at the possibility that hydrogen-dependent life could have existed where iron-rich igneous rocks on Mars were once in contact with water.
May 30, 2013 — A chemical reaction between iron-containing minerals and water may produce enough hydrogen "food" to sustain microbial communities living in pores and cracks within the enormous volume of rock below the ocean floor and parts of the continents, according to a new study led by the University of Colorado Boulder.Mars surface. A chemical reaction between iron-containing minerals and water may produce enough hydrogen "food" to sustain microbial communities living in pores and cracks within the enormous volume of rock below the ocean floor and parts of the continents, according to a new study. The findings also hint at the possibility that hydrogen-dependent life could have existed where iron-rich igneous rocks on Mars were once in contact with water.
The findings, published in the journal Nature Geoscience, also hint at the possibility that hydrogen-dependent life could have existed where iron-rich igneous rocks on Mars were once in contact with water.
Scientists have thoroughly investigated how rock-water reactions can produce hydrogen in places where the temperatures are far too hot for living things to survive, such as in the rocks that underlie hydrothermal vent systems on the floor of the Atlantic Ocean. The hydrogen gases produced in those rocks do eventually feed microbial life, but the communities are located only in small, cooler oases where the vent fluids mix with seawater.
The new study, led by CU-Boulder Research Associate Lisa Mayhew, set out to investigate whether hydrogen-producing reactions also could take place in the much more abundant rocks that are infiltrated with water at temperatures cool enough for life to survive.
"Water-rock reactions that produce hydrogen gas are thought to have been one of the earliest sources of energy for life on Earth," said Mayhew, who worked on the study as a doctoral student in CU-Boulder Associate Professor Alexis Templeton's lab in the Department of Geological Sciences.
"However, we know very little about the possibility that hydrogen will be produced from these reactions when the temperatures are low enough that life can survive. If these reactions could make enough hydrogen at these low temperatures, then microorganisms might be able to live in the rocks where this reaction occurs, which could potentially be a huge subsurface microbial habitat for hydrogen-utilizing life."
When igneous rocks, which form when magma slowly cools deep within Earth, are infiltrated by ocean water, some of the minerals release unstable atoms of iron into the water. At high temperatures -- warmer than 392 degrees Fahrenheit (200 degrees Celsius) -- scientists know that the unstable atoms, known as reduced iron, can rapidly split water molecules and produce hydrogen gas, as well as new minerals containing iron in the more stable, oxidized form.
Mayhew and her co-authors, including Templeton, submerged rocks in water in the absence of oxygen to determine if a similar reaction would take place at much lower temperatures, between 122 and 212 degrees Fahrenheit (50 to 100 degrees Celsius). The researchers found that the rocks did create hydrogen -- potentially enough hydrogen to support life.
To understand in more detail the chemical reactions that produced the hydrogen in the lab experiments, the researchers used "synchrotron radiation" -- which is created by electrons orbiting in a humanmade storage ring -- to determine the type and location of iron in the rocks on a microscale.
The researchers expected to find that the reduced iron in minerals like olivine had converted to the more stable oxidized state, just as occurs at higher temperatures. But when they conducted their analyses at the Stanford Synchrotron Radiation Lightsource at Stanford University, they were surprised to find newly formed oxidized iron on "spinel" minerals found in the rocks. Spinels are minerals with a cubic structure that are highly conductive.
Finding oxidized iron on the spinels led the team to hypothesize that, at low temperatures, the conductive spinels were helping facilitate the exchange of electrons between reduced iron and water, a process that is necessary for the iron to split the water molecules and create the hydrogen gas.
"After observing the formation of oxidized iron on spinels, we realized there was a strong correlation between the amount of hydrogen produced and the volume percent of spinel phases in the reaction materials," Mayhew said. "Generally, the more spinels, the more hydrogen."
Not only is there a potentially large volume of rock on Earth that may undergo these low temperature reactions, but the same types of rocks also are prevalent on Mars, Mayhew said. Minerals that form as a result of the water-rock reactions on Earth have been detected on Mars as well, which means that the process described in the new study may have implications for potential Martian microbial habitats.
Mayhew and Templeton are already building on this study with their co-authors, including Thomas McCollom at CU-Boulder's Laboratory for Atmospheric and Space Physics, to see if the hydrogen-producing reactions can actually sustain microbes in the lab.
This study was funded by the David and Lucille Packard Foundation and with a U.S. Department of Energy Early Career grant to Templeton.
奈米科技簡化氫氣產量使之成為乾淨能源
Nanotechnology Simplifies Hydrogen Production for Clean Energy
Nov. 20, 2012 — In the first-ever experiment of its kind, researchers have demonstrated that clean energy hydrogen can be produced from water splitting by using very small metal particles that are exposed to sunlight.
In the article, "Outstanding activity of sub-nm Au clusters for photocatalytic hydrogen production," published in the journal Applied Catalysis B: Environmental, Alexander Orlov, PhD, an Assistant Professor of Materials Science & Engineering at Stony Brook University, and his colleagues from Stony Brook and Brookhaven National Laboratory, found that the use of gold particles smaller than one nanometer resulted in greater hydrogen production than other co-catalysts tested.
"This is the first ever demonstration of the remarkable potential of very small metal nanoparticles [containing fewer than a dozen atoms] for making fuel from water," said Professor Orlov. Using nanotechnology, Professor Orlov's group found that when the size of metal particles are reduced to dimensions below one nanometer, there is a tremendous increase in the ability of these particles to facilitate hydrogen production from water using solar light. They observed a "greater than 35 times increase" in hydrogen evolution as compared to ordinary materials.
In order to explain these fascinating results, Professor Orlov collaborated with Brookhaven National Lab computational scientist Dr. Yan Li, who found some interesting anomalies in electronic properties of these small particles. Professor Orlov noted that there is still a tremendous amount of work that needs be done to understand this phenomenon. "It is conceivable that we are only at the beginning of an extraordinary journey to utilize such small particles [of less than a dozen atoms in size] for clean energy production," he said.
"In order to reduce our dependence on fossil fuels it is vital to explore various sustainable energy options," Professor Orlov said. "One possible strategy is to develop a hydrogen-based energy economy, which can potentially offer numerous environmental and energy efficiency benefits. Hydrogen can conceivably be a promising energy source in the future as it is a very clean fuel, which produces water as a final combustion product. The current challenge is to find new materials, which can help to produce hydrogen from sustainable sources, such as water."
Professor Orlov also serves as a faculty member of the Consortium for Inter-Disciplinary Environmental Research at Stony Brook University. Members of his research team include Peichuan Shen and Shen Zhao from the Department of Materials Science and Engineering at Stony Brook and Dr. Dong Su of the Center for Functional Nanomaterials at Brookhaven National Laboratory.

Experimental and theory predicted optical properties of supported sub-nanometer particles.
(Credit: Image courtesy of Stony Brook University)
地熱利用:
全台首座地熱電廠 發電達30佰萬瓦

宜蘭縣政府撥用清水地熱區約13公頃土地,規劃運用地熱資源,希望建立具教育、環保及綠能的地熱能源生態園區。(攝影:曾漢東/大紀元)
宜蘭縣政府昨(17)日正式與噶瑪蘭清水股份有限公司簽約,為宜蘭縣清水地熱發電整建、營運、移轉(ROT)案定調,也預期在未來12個月內完成1MW發電量電廠整建工程,打造全台灣第一座商業運轉的地熱發電廠。
台灣座落於地熱資源主要分布區、環太平洋兩岸的火環帶,全島淺層地熱預計有近1,000佰萬瓦之發電潛能,而清水地區,初步的發電量評估約為10至30佰萬瓦。
噶瑪蘭清水(地熱發電)股份有限公司看中清水地區的地熱發電潛力,引進世界三大航空發動機製造商,普惠(Pratt & Whitney)所打造的Pure cycle 280機組,將為台灣建造第一個示範性潔淨能源電廠,並以全台地熱資源整體開發及成為亞洲模範生與綠能標竿為長期願景。
宜蘭縣政府指出,清水地熱水量豐沛,深具地熱開發潛能;縣府於民國90年初,撥用清水地熱區約13公頃後,即開始規劃運用地熱資源,希望建立具教育、環保及綠能的地熱能源生態園區。清水地熱發電整建、營運、移轉(ROT)案簽約儀式後,未來的清水地熱可以重新發電,發揮最大功效。
台灣座落於地熱資源主要分布區、環太平洋兩岸的火環帶,全島淺層地熱預計有近1,000佰萬瓦之發電潛能,而清水地區,初步的發電量評估約為10至30佰萬瓦。
噶瑪蘭清水(地熱發電)股份有限公司看中清水地區的地熱發電潛力,引進世界三大航空發動機製造商,普惠(Pratt & Whitney)所打造的Pure cycle 280機組,將為台灣建造第一個示範性潔淨能源電廠,並以全台地熱資源整體開發及成為亞洲模範生與綠能標竿為長期願景。
宜蘭縣政府指出,清水地熱水量豐沛,深具地熱開發潛能;縣府於民國90年初,撥用清水地熱區約13公頃後,即開始規劃運用地熱資源,希望建立具教育、環保及綠能的地熱能源生態園區。清水地熱發電整建、營運、移轉(ROT)案簽約儀式後,未來的清水地熱可以重新發電,發揮最大功效。
台灣地熱發電量=2.7座核四 !!! ???
3月11日,日本東北外海發生史上第五大、規模達9.0的地震,引發10公尺海嘯不僅摧毀日本東北幾個臨海縣市,更造成核電廠災變導致輻射線外洩。此事件勾起全世界對於輻射的恐慌,核電廠的安全性成為眾所注目的焦點,其必要性也再被質疑。同處於地震帶的台灣,目前有三座運轉中的核電廠,不僅都位於活動斷層帶附近,且皆已老舊接近除役年份,如何開發替代性的再生能源,是目前的當務之急。最近報章媒體談論的再生能源不外乎風力、太陽能、洋流和生質能源,鮮少人提及台灣擁有相當豐富的地熱能,故筆者撰文淺談之。
地球為一個巨大的散熱體,時時刻刻源源不絕地散出豐富熱能,地表每年散失的熱量高達2.4x1017千卡,約相當於燃燒1.68X1010桶石油,也相當於全世界一年所消耗能源的43%。過去三十年,隨著材料和技術的進步,世界各國的地熱資源發展相當快,約增加6-7倍(從1,300增長至9,450佰萬瓦),且持續快速增加中,預計2020年可達到30,000-40,000佰萬瓦,是目前的3至4倍。美國能源部於公元2000年便開始推動Geopowering the West的計畫,預計於2020年地熱能可達20,000佰萬瓦;我們鄰近的菲律賓,在美國的技術和資金的支助下,積極的開發地熱資源,目前已經裝載了1,931佰萬瓦的發電量(佔該國總發電量的13%),僅次於美國,成為世界第二大地熱發電的國家,估計未來的十年內其地熱發電潛能可達4,335佰萬瓦。美國歐巴馬總統2008年上任後即通過3億5千萬美元的預算,用於地熱能源相關技術的開發,網路巨人Google公司也加入地熱能源方面的投資,向Alta Rock和Potter Drilling兩家地熱公司各投資625萬和400萬美元。
相對於傳統淺層地熱能之利用,蘊藏於更深部地層(3,000-10,000公尺)之熱能更是龐大,且較不受地域限制;美國麻省理工學院Tester教授等人的估計,全球深層地熱發電潛能約為地球石化燃料發電潛能的300倍,並倡議使用「地熱加強生產系統」擴大傳統地熱生產的規模,以開採利用3,000公尺以下或更深層的地熱能源,以取代快速耗竭的石化能源。
全世界地熱資源主要分布於環太平洋兩岸的火環帶,而台灣正是座落此區,所以有豐富的地熱資源。根據1980年以前之探勘及普查資料估算,全島淺層地熱預計有近1,000佰萬瓦之發電潛能。1981年所建的清水地熱發電廠,曾使台灣成為全球第十四個地熱發電的國家。但因地熱井結垢阻塞和機組選擇不當,而於1993年關廠,甚為可惜。
近年來因探勘技術進步,初步研究顯示台灣整個中央山脈都是地熱高區,尤其是北部宜蘭清水和東南部知本兩地區。另外,北部的大屯火山群更蘊藏比前面兩地區還高的地熱量。台灣地區可發電量以清水地區為例,傳統地熱徵兆區約有1.4平分公里,初步的發電量評估約為10至30佰萬瓦。而其鄰近宜蘭地區廣約100平方公里的地底下都有相似於清水地區的地熱蘊藏量,故估計其可能的發電量可高達700至2,100佰萬瓦。
相對於傳統淺層地熱能之利用,蘊藏於更深部地層(3,000-5,000公尺)之熱能更是龐大。故國科會在2008年所推動能源型國家科技計畫報告書中,更估計台灣地熱的發電量可達7,100佰萬瓦,相當於2.7座核四廠(發電量為2,600佰萬瓦)的發電量。
地熱能源在台灣具有廣泛的分布、不排放二氧化碳對環境友善和獨特性、不受氣候和其他能源發展的影響、運轉率高(平均可達90%以上)又可作為基載電力,且有多方面的應用效能,以及具有永續發展等特性,符合現今再生能源的發展趨勢,是一種極具開發潛力、應用於發電和直接利用(包括地熱唧筒、空間加熱、溫室加熱、水產養殖、農產品乾燥加工、泡湯和家用冷卻和加溫等)的再生能源。若能善加利用,不僅可減少對化石能源的依賴和達減碳的效果,更有益於能源開發應用之多元化與自主性。
(作者為國立台灣大學地質科學系教授)
3月11日,日本東北外海發生史上第五大、規模達9.0的地震,引發10公尺海嘯不僅摧毀日本東北幾個臨海縣市,更造成核電廠災變導致輻射線外洩。此事件勾起全世界對於輻射的恐慌,核電廠的安全性成為眾所注目的焦點,其必要性也再被質疑。同處於地震帶的台灣,目前有三座運轉中的核電廠,不僅都位於活動斷層帶附近,且皆已老舊接近除役年份,如何開發替代性的再生能源,是目前的當務之急。最近報章媒體談論的再生能源不外乎風力、太陽能、洋流和生質能源,鮮少人提及台灣擁有相當豐富的地熱能,故筆者撰文淺談之。
地球為一個巨大的散熱體,時時刻刻源源不絕地散出豐富熱能,地表每年散失的熱量高達2.4x1017千卡,約相當於燃燒1.68X1010桶石油,也相當於全世界一年所消耗能源的43%。過去三十年,隨著材料和技術的進步,世界各國的地熱資源發展相當快,約增加6-7倍(從1,300增長至9,450佰萬瓦),且持續快速增加中,預計2020年可達到30,000-40,000佰萬瓦,是目前的3至4倍。美國能源部於公元2000年便開始推動Geopowering the West的計畫,預計於2020年地熱能可達20,000佰萬瓦;我們鄰近的菲律賓,在美國的技術和資金的支助下,積極的開發地熱資源,目前已經裝載了1,931佰萬瓦的發電量(佔該國總發電量的13%),僅次於美國,成為世界第二大地熱發電的國家,估計未來的十年內其地熱發電潛能可達4,335佰萬瓦。美國歐巴馬總統2008年上任後即通過3億5千萬美元的預算,用於地熱能源相關技術的開發,網路巨人Google公司也加入地熱能源方面的投資,向Alta Rock和Potter Drilling兩家地熱公司各投資625萬和400萬美元。
相對於傳統淺層地熱能之利用,蘊藏於更深部地層(3,000-10,000公尺)之熱能更是龐大,且較不受地域限制;美國麻省理工學院Tester教授等人的估計,全球深層地熱發電潛能約為地球石化燃料發電潛能的300倍,並倡議使用「地熱加強生產系統」擴大傳統地熱生產的規模,以開採利用3,000公尺以下或更深層的地熱能源,以取代快速耗竭的石化能源。
全世界地熱資源主要分布於環太平洋兩岸的火環帶,而台灣正是座落此區,所以有豐富的地熱資源。根據1980年以前之探勘及普查資料估算,全島淺層地熱預計有近1,000佰萬瓦之發電潛能。1981年所建的清水地熱發電廠,曾使台灣成為全球第十四個地熱發電的國家。但因地熱井結垢阻塞和機組選擇不當,而於1993年關廠,甚為可惜。
近年來因探勘技術進步,初步研究顯示台灣整個中央山脈都是地熱高區,尤其是北部宜蘭清水和東南部知本兩地區。另外,北部的大屯火山群更蘊藏比前面兩地區還高的地熱量。台灣地區可發電量以清水地區為例,傳統地熱徵兆區約有1.4平分公里,初步的發電量評估約為10至30佰萬瓦。而其鄰近宜蘭地區廣約100平方公里的地底下都有相似於清水地區的地熱蘊藏量,故估計其可能的發電量可高達700至2,100佰萬瓦。
相對於傳統淺層地熱能之利用,蘊藏於更深部地層(3,000-5,000公尺)之熱能更是龐大。故國科會在2008年所推動能源型國家科技計畫報告書中,更估計台灣地熱的發電量可達7,100佰萬瓦,相當於2.7座核四廠(發電量為2,600佰萬瓦)的發電量。
地熱能源在台灣具有廣泛的分布、不排放二氧化碳對環境友善和獨特性、不受氣候和其他能源發展的影響、運轉率高(平均可達90%以上)又可作為基載電力,且有多方面的應用效能,以及具有永續發展等特性,符合現今再生能源的發展趨勢,是一種極具開發潛力、應用於發電和直接利用(包括地熱唧筒、空間加熱、溫室加熱、水產養殖、農產品乾燥加工、泡湯和家用冷卻和加溫等)的再生能源。若能善加利用,不僅可減少對化石能源的依賴和達減碳的效果,更有益於能源開發應用之多元化與自主性。
(作者為國立台灣大學地質科學系教授)

沒有留言:
張貼留言
注意:只有此網誌的成員可以留言。